Feb 15, 2023Chemistry Ashtrid A. asked • 02/15/23 Pressure of each gas after equilibrium Consider the following reaction. 2NO2 (g)⇌N2O4 (g) When the system is at equilibrium, it contains NO2 at a pressure of 0.835 atm, and N2O4 at a pressure of 0.0697 atm. The volume of the container is then reduced to half its original volume.
The Effect of Pressure on Equilibrium – N2O4 to 2NO2 – YouTube
Find stepby-step Chemistry solutions and your answer to the following textbook question: The partial pressures of an equilibrium mixture of $\ceN2O4 (g)$ and $\ceNO2 (g)$ are $\ceP_N_2O_4$ = 0.35 atm and $\ceP_NO_2$ = 1.15 atm at a certain temperature. The volume of the container is doubled. Find the partial pressures of the two gases when a new equilibrium is established.
Oct 17, 2023When a gas mixture is prepared at 600K, in which 9.6 atm is the initial partial pressure of both NO 2 and CO, and 5.4 atm is the partial pressure of both NO and CO 2, the brown color of the mixture observed begins to get stronger as the reaction progresses toward equilibrium. Give a condition that must be satisfied by the equilibrium constant K.

Source Image: researchgate.net
Download Image
Equilibrium partial pressure of gaseous decomposition products of… | Download Scientific Diagram Interpretation: To Calculate and analyze the each partial pressure (Kp) values of given N2O4 into NO2 equilibrium reaction with respective temperature at 25 ο C.. Concept Introduction: Equilibrium constant: Concentration of the products to the respective molar concentration of reactants it is called equilibrium constant. If the K value is less than one the reaction will move to the left side

Source Image: m.youtube.com
Download Image
What Is The Partial Pressure Of N2o4 At Equilibrium
Interpretation: To Calculate and analyze the each partial pressure (Kp) values of given N2O4 into NO2 equilibrium reaction with respective temperature at 25 ο C.. Concept Introduction: Equilibrium constant: Concentration of the products to the respective molar concentration of reactants it is called equilibrium constant. If the K value is less than one the reaction will move to the left side Write a description of the anticipatory socialization you went through in preparation for adolescent. Find step-by-step Chemistry solutions and your answer to the following textbook question: At 25 degree C, the equilibrium partial pressures of NO2 and N2O4 are 0.15 atm and 0.20 atm, respectively. If the volume is doubled at constant
The Effect of Pressure on Equilibrium – N2O4 to 2NO2 – YouTube
Jan 30, 2023Solution. First, calculate the partial pressure for H2O H 2 O by subtracting the partial pressure of H2 H 2 from the total pressure. PH2O = Ptotal −PH2 = (0.016 − 0.013) atm = 0.003 atm P H 2 O = P t o t a l − P H 2 = ( 0.016 − 0.013) a t m = 0.003 a t m. Then, write K (equilibrium constant expression) in terms of activities. Equilibrium, Calculating Kc. N2O4 (g) ⇌ 2NO2(g) – YouTube
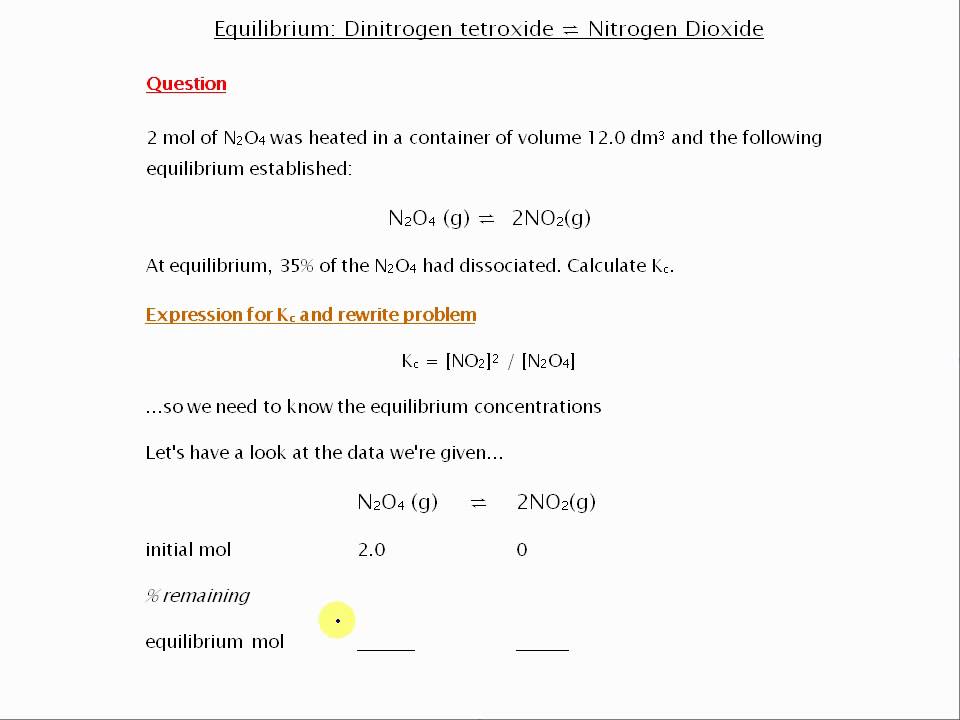
Source Image: youtube.com
Download Image
Principles of Chemical Equilibrium Jan 30, 2023Solution. First, calculate the partial pressure for H2O H 2 O by subtracting the partial pressure of H2 H 2 from the total pressure. PH2O = Ptotal −PH2 = (0.016 − 0.013) atm = 0.003 atm P H 2 O = P t o t a l − P H 2 = ( 0.016 − 0.013) a t m = 0.003 a t m. Then, write K (equilibrium constant expression) in terms of activities.
Source Image: acikders.ankara.edu.tr
Download Image
The Effect of Pressure on Equilibrium – N2O4 to 2NO2 – YouTube Feb 15, 2023Chemistry Ashtrid A. asked • 02/15/23 Pressure of each gas after equilibrium Consider the following reaction. 2NO2 (g)⇌N2O4 (g) When the system is at equilibrium, it contains NO2 at a pressure of 0.835 atm, and N2O4 at a pressure of 0.0697 atm. The volume of the container is then reduced to half its original volume.

Source Image: m.youtube.com
Download Image
Equilibrium partial pressure of gaseous decomposition products of… | Download Scientific Diagram Oct 17, 2023When a gas mixture is prepared at 600K, in which 9.6 atm is the initial partial pressure of both NO 2 and CO, and 5.4 atm is the partial pressure of both NO and CO 2, the brown color of the mixture observed begins to get stronger as the reaction progresses toward equilibrium. Give a condition that must be satisfied by the equilibrium constant K.

Source Image: researchgate.net
Download Image
OneClass: What is the equilibrium partial pressure of N2O4? Value of Kp of the reaction, calculate th… VIDEO ANSWER: A reaction consists of 2 n o gaseous and 4 n o gaseous. The gases are given partial pressures. The measurement is 0.258 m and 4 n 2 o 4. The product concentration is 4.38 m a t m, divided by the partial…

Source Image: oneclass.com
Download Image
20.An equilibrium mixture at 300K contains N2O4 and NO2 at 0.28 and 1.1 atm pressure respectively. If the volume of the container is doubled, calculate the new equilibrium pressure of two gases Interpretation: To Calculate and analyze the each partial pressure (Kp) values of given N2O4 into NO2 equilibrium reaction with respective temperature at 25 ο C.. Concept Introduction: Equilibrium constant: Concentration of the products to the respective molar concentration of reactants it is called equilibrium constant. If the K value is less than one the reaction will move to the left side
Source Image: byjus.com
Download Image
Equilibrium partial pressure of gaseous decomposition products of… | Download Scientific Diagram Write a description of the anticipatory socialization you went through in preparation for adolescent. Find step-by-step Chemistry solutions and your answer to the following textbook question: At 25 degree C, the equilibrium partial pressures of NO2 and N2O4 are 0.15 atm and 0.20 atm, respectively. If the volume is doubled at constant

Source Image: researchgate.net
Download Image
Principles of Chemical Equilibrium
Equilibrium partial pressure of gaseous decomposition products of… | Download Scientific Diagram Find stepby-step Chemistry solutions and your answer to the following textbook question: The partial pressures of an equilibrium mixture of $\ceN2O4 (g)$ and $\ceNO2 (g)$ are $\ceP_N_2O_4$ = 0.35 atm and $\ceP_NO_2$ = 1.15 atm at a certain temperature. The volume of the container is doubled. Find the partial pressures of the two gases when a new equilibrium is established.
Equilibrium partial pressure of gaseous decomposition products of… | Download Scientific Diagram 20.An equilibrium mixture at 300K contains N2O4 and NO2 at 0.28 and 1.1 atm pressure respectively. If the volume of the container is doubled, calculate the new equilibrium pressure of two gases VIDEO ANSWER: A reaction consists of 2 n o gaseous and 4 n o gaseous. The gases are given partial pressures. The measurement is 0.258 m and 4 n 2 o 4. The product concentration is 4.38 m a t m, divided by the partial…